Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)

Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)

Long‐term efficacy and safety results from an open‐label phase III study (UNCOVER‐J) in Japanese plaque psoriasis patients: impact of treatment withdrawal and retreatment of ixekizumab - Umezawa - 2019 - Journal of
Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials | SpringerLink
![PDF] Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience | Semantic Scholar PDF] Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8475108e210007edf6f24a80c253692db913891f/2-Figure1-1.png)
PDF] Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience | Semantic Scholar

PDF) Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: Results from the randomised, controlled and open-label phases of UNCOVER-3

LB0005 MULTICENTRE, RANDOMISED, OPEN-LABEL, ASSESSOR-BLINDED, PARALLEL-GROUP HEAD-TO-HEAD COMPARISON OF THE EFFICACY AND SAFETY OF IXEKIZUMAB VERSUS ADALIMUMAB IN PATIENTS WITH PSORIATIC ARTHRITIS NAIVE TO BIOLOGIC DISEASE-MODIFYING ANTI-RHEUMATIC ...

These highlights do not include all the information needed to use TALTZ safely and effectively. See full prescribing information for TALTZ.TALTZ ( ixekizumab) injection, for subcutaneous useInitial U.S. Approval: 2016

Lilly's Taltz® (ixekizumab) is the First IL-17A Antagonist to Receive U.S. FDA Approval for the Treatment of Non-Radiographic Axial Spondyloarthritis (nr-axSpA)

Figure 1. | Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis | The Journal of Rheumatology

Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial - The Lancet

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - Journal of the American Academy of Dermatology

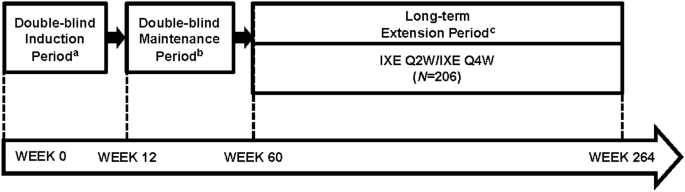
Study design for administration of ixekizumab via a prefilled syringe... | Download Scientific Diagram